Cold and ultracold molecules
Our first experiment focuses on the control of molecules on the single quantum state level. Molecules are interesting because compared to atoms, they have extra internal and external degrees of freedom, lending them rich internal structure and long-range anisotropic interactions. To get into the quantum regime, we will develop new methods to cool and control molecules. The well-controlled molecular building blocks can then be harnessed for explorations of new condensed matter phases, quantum chemistry studies, fundamental physics measurements, quantum information processing, and more.
A sample of previous research on cold and ultracold molecules in bulk gases can be found below.
Ultracold polar molecules
Despite the promise of ultracold polar molecules, molecules have traditionally been challenging to cool and control, primarily due to their vibrational degree of freedom leading to a lack of closed cycling transitions. One way to get around this problem is to first cool atoms, and then form molecules from the ultracold atoms.
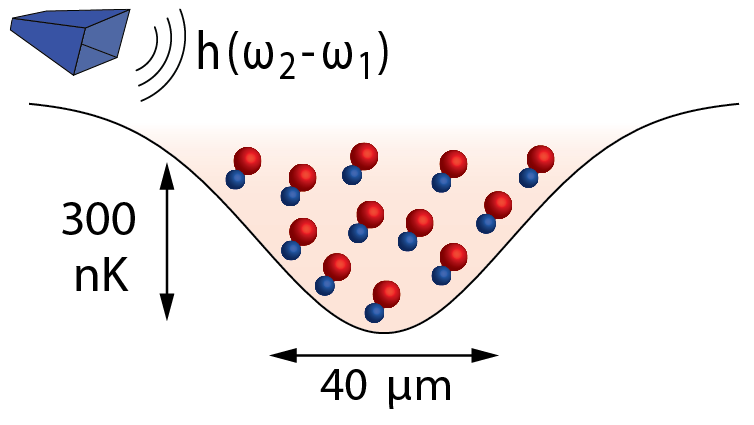
spin-polarized ultracold molecules
The creation of a high phase-space density of ultracold polar molecules was pioneered at JILA using potassium rubidium (KRb). This was also later achieved at MIT, where we
formed sodium potassium (NaK) molecules that are spin-polarized in the ground quantum state. To do this, we first magnetoassociated ultracold samples of Na and K atoms to form loosely bound Feshbach molecules, then coherently transferred them to the ground molecular state using stable laser. The ground state molecules, as cold as 250 nK, retain the coherence of the atomic sample from which they are created.
To engineer long-range dipolar interactions, one can apply microwaves to mix the lowest and first excited rotational states. In this paper, we demonstrated microwave control over the two lowest rotational states. Going beyond rotational states, we have also observed nuclear spin coherence times on the scale of 1 second, which opens the door to using these molecules for quantum memory and high-resolution, Hertz-level spectroscopy.
Cold molecular ions for an electron electric dipole moment search
electric field. Credit: JILA
Precision spectroscopy of molecules can facilitate the search for physics beyond the Standard Model. For instance, new physics predicts that the electron electric dipole moment (eEDM) may be up to ten orders of magnitude larger than that predicted by the Standard Model. One can look for an electron electric dipole moment (eEDM) by measuring tiny frequency shifts arising from the eEDM interacting with an electric field.
Candidate molecules like the hafnium fluoride molecular ion have been proposed to be well-suited for such a precision eEDM search, because:
- The internal effective electric field can be as large as about 20 GV/cm;
- Clever combinations of spectroscopic transitions can be used to cancel out systematic frequency shifts;
- Being ions, the molecules can be easily trapped by electromagnetic fields in a radiofrequency Paul trap and interrogated for a long time.
Such precision spectroscopy demands the ability to prepare, manipulate, and detect the molecular ions’ quantum states with high efficiency. In this paper, we combine the tools we developed to control the hafnium fluoride molecular ions and present coherent Ramsey spectroscopy at the 10 Hz level, where the ions’ Berry phase was exploited as a spectroscopic tool.